Trending...
- Triple-Digit Growth, Strategic N A S D A Q Uplist, Plus A Scalable Healthcare Rollout Model: Stock Symbol: CDIX
- 59-Acre Germantown Horse Farm Sells for $1.3 Million
- Ludex Partners With Certified Trading Card Association (CTCA) To Elevate Standards And Innovation In The Trading Card Industry
FAYETTEVILLE, Ark. - WisconsinEagle -- Lineus Medical is now officially registered in the United Kingdom, enabling the company to begin distributing SafeBreak® Vascular within the UK healthcare market. UK registration represents an important step in Lineus Medical's international expansion strategy, further extending access to SafeBreak Vascular for hospitals and clinicians on a global scale. With regulatory requirements in place, Lineus Medical can now work with distribution partners to bring its breakaway IV technology to the UK.
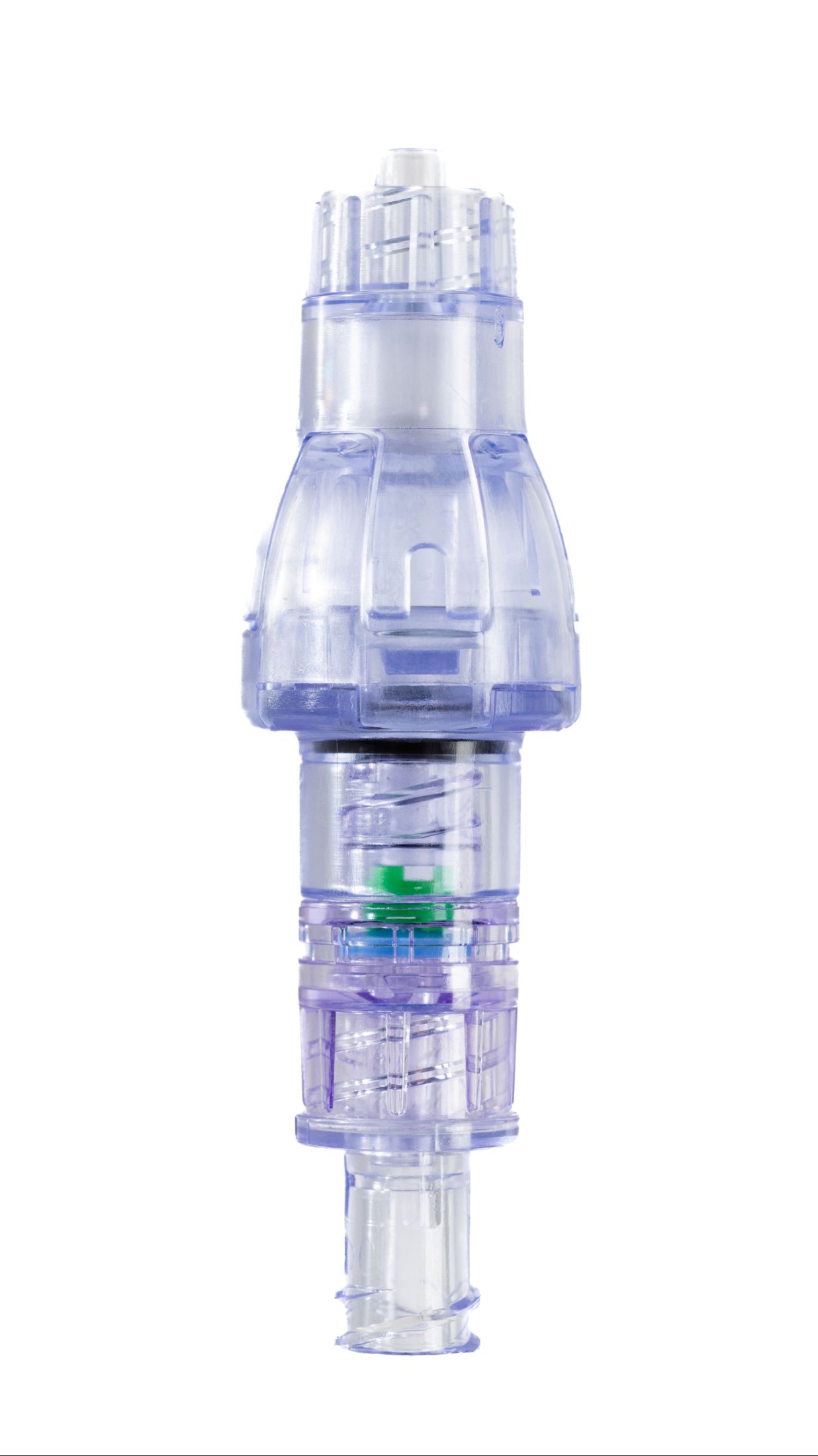
SafeBreak Vascular is a breakaway device for IV lines clinically proven to reduce IV complications.¹ When a harmful force is placed on the line, SafeBreak intentionally separates to remove the damaging force and protect the patient's IV. When separation occurs, valves on both ends of the device close to prevent medication spills from the pump and blood loss from the patient. Patients avoid additional needlesticks, nurses save time, and hospitals save money.¹
More on Wisconsin Eagle
"Completing UK registration is another meaningful milestone as we continue to expand access to SafeBreak globally," said Vance Clement, CEO of Lineus Medical. "Each new market brings us closer to our mission of removing the pains associated with IV lines and improving patient safety across healthcare systems worldwide. UK registration allows SafeBreak to reach another 69 million people."
"This registration allows us to move forward with distribution planning in the UK and supports our broader international commercialization efforts," said Larry Hayes, Chief Commercial Officer of Lineus Medical. "We are focused on working with the right partners to ensure SafeBreak Vascular is accessible to clinicians who are looking to reduce IV complications and improve care at the bedside."
About Lineus Medical:
Lineus Medical is the developer of SafeBreak® Vascular, a breakaway technology proven to reduce IV restarts and IV complications.1 Our mission is to remove the pains associated with medical lines. More information about Lineus Medical can be found at www.lineusmed.com. Follow Lineus Medical on LinkedIn, Facebook, and Instagram.
References
SafeBreak Vascular is a breakaway device for IV lines clinically proven to reduce IV complications.¹ When a harmful force is placed on the line, SafeBreak intentionally separates to remove the damaging force and protect the patient's IV. When separation occurs, valves on both ends of the device close to prevent medication spills from the pump and blood loss from the patient. Patients avoid additional needlesticks, nurses save time, and hospitals save money.¹
More on Wisconsin Eagle
- Distributed Social Media - Own Your Content
- Tarrytown Expocare Pharmacy Announces Strategic Leadership Appointments to Accelerate Growth and Innovation
- New Environmental Thriller "The Star Thrower" Reimagines a Classic Lesson in Individual Impact
- Summit Appoints Javier Cabeza as Data, AI, and Analytics Practice Lead
- March Is Skiing's Smartest Buying Window
"Completing UK registration is another meaningful milestone as we continue to expand access to SafeBreak globally," said Vance Clement, CEO of Lineus Medical. "Each new market brings us closer to our mission of removing the pains associated with IV lines and improving patient safety across healthcare systems worldwide. UK registration allows SafeBreak to reach another 69 million people."
"This registration allows us to move forward with distribution planning in the UK and supports our broader international commercialization efforts," said Larry Hayes, Chief Commercial Officer of Lineus Medical. "We are focused on working with the right partners to ensure SafeBreak Vascular is accessible to clinicians who are looking to reduce IV complications and improve care at the bedside."
About Lineus Medical:
Lineus Medical is the developer of SafeBreak® Vascular, a breakaway technology proven to reduce IV restarts and IV complications.1 Our mission is to remove the pains associated with medical lines. More information about Lineus Medical can be found at www.lineusmed.com. Follow Lineus Medical on LinkedIn, Facebook, and Instagram.
References
- Data on file.
Source: Lineus Medical
0 Comments
Latest on Wisconsin Eagle
- Progressive Dental & The Closing Institute Partner with Zest Dental Solutions to Elevate Full-Arch Growth and Patient Outcomes
- CCHR: While Damaging Antipsychotics Win Approval, Proven Non-Drug Alternatives Remain Ignored
- Arcuri Group Announces Long‑Term Partnership with WakeMed Health & Hospitals to Deliver Situational Awareness and De‑escalation Training
- At 25, She Became One of the Youngest AAPI Female Founders to Win One of the World's Most Prestigious Design Awards for a Lamp That Makes You Smile
- Juego Studios Extends Full-Cycle Game Development & Outsourcing Capabilities to the UAE Market
- VENUS Goes Live on CATEX Exchange As UK Financial Ltd Activates The Premier Division Of The Maya Meme's League
- Atlanta Tech Founder Seeks Clarity on Intellectual Property and Innovation Policy
- Purple Heart Recipient Honored by Hall of Fame Son In Viral Tribute Sparking National Conversation on Service Fatherhood, Healing and Legacy
- Amicly Launches as a Safety-First Social App Designed to Help People Build Real, Meaningful Friendships
- Primeindexer Google indexing platform launched by SEO Danmark APS
- Kaltra Introduces New Downward-Spraying Distribution Technology to Boost Microchannel Evaporator Performance
- Talentica Announces Winners of Multi-Agent Hackathon 2026
- NeuroAcoustic Pioneer Dr. Chris Potter Unveils QLL Technology to Disrupt Mental Health Industry
- Special Alert: Undervalued Opportunity: IQSTEL (N A S D A Q: IQST) Positioned for Explosive Multi-Year Growth
- Triple-Digit Growth, Strategic N A S D A Q Uplist, Plus A Scalable Healthcare Rollout Model: Stock Symbol: CDIX
- Vesica Health Receives FDA Breakthrough Device Designation for AssureMDx
- Lineus Medical's SafeBreak® Vascular Added to Alliant GPO Contract
- Cancun All Inclusive is ready for Spring Break 2026 with new Resorts, Exclusive Deals, activities and more!
- 66% of US Bankruptcies Are Medical — So Americans Are Building Businesses That Cover Healthcare Emergencies
- Ludex Partners With Certified Trading Card Association (CTCA) To Elevate Standards And Innovation In The Trading Card Industry