Trending...
- Hiclean Tools Releases HCX2100 Electric Pressure Washer
- Some Music for Donald's Bad Day
- America Anesthesia Partners Unveils New User-Friendly Website
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) $NRXP Refiles Abbreviated New Drug Application; $40 Price Target in New H. C. Wainright Research Report
MIAMI - WisconsinEagle -- Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
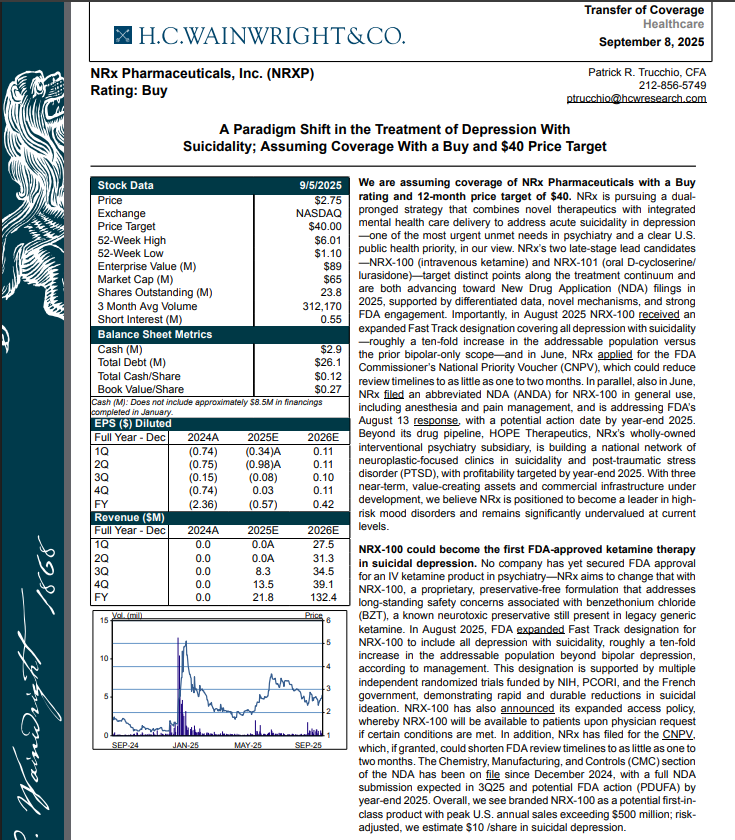
H.C. Wainright Analyst Report Cites Paradigm Shift in the Treatment of Depression with Suicidality; Assuming Coverage with Buy and $40 Price Target.
Re-Filing of Abbreviated New Drug Application (ANDA) for KETAFREE™, Preservative-Free IV Ketamine.
Notification of US Food and Drug Administration Approval of Suitability Petition for NRx's Proposed Strength of Preservative-Free Ketamine.
Current Ketamine Market Estimated at $750 Million and Projected to Reach $3.35 Billion Globally in 2034.
Dura Medical Acquisition Completed in Network of Interventional Psychiatry Clinics.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
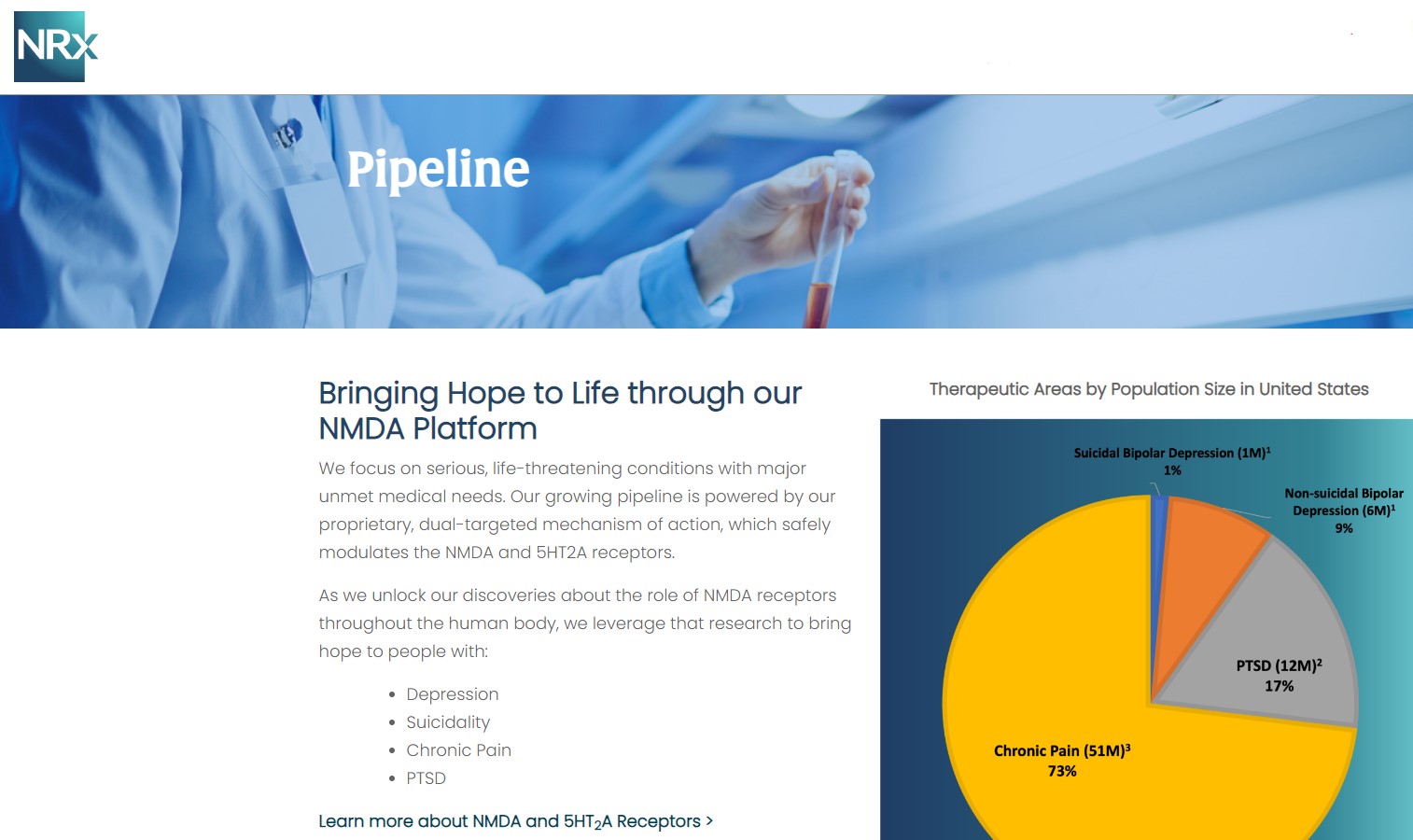
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
H.C. Wainwright has issued a new Analyst Report on NRXP: "A Paradigm Shift in the Treatment of Depression With Suicidality" Assuming Coverage With a Buy and $40 Price Target. The full report may be accessed at this direct link: https://hcwco.bluematrix.com/links2/secure/pdf/acdd3260-630e-48e6-9c2f-03fbc0be37d6
More on Wisconsin Eagle
NRXP Re-Files Abbreviated New Drug Application (ANDA) for KETAFREE™, Preservative-Free IV Ketamine
On September 29th NRXP announced the re-filing of its Abbreviated New Drug Application (ANDA) to the U.S. Food and Drug Administration (FDA) for KETAFREE™, its preservative-free IV ketamine formulation, for use in all existing approved indications. The filing follows FDA grant of approval of its Suitability Petition for the NRXP proposed strength of preservative-free ketamine.
The current annual ketamine market is estimated at $750 million, with global demand for ketamine projected to grow to $3.35 billion by 2034. This does not include the widespread use of compounded ketamine by clinics unable to obtain manufactured drug. NRx aims to capture a significant share of the current market. According to a 2021 survey, an estimated 5.1 million Americans had received ketamine for medical uses in their lifetime3, a number that continues to grow with increased clinical focus on this important medication. Ketamine currently faces a severe drug shortage according to the American Society of Hospital Pharmacists. Accordingly, NRXP is seeking priority review from FDA.
NRXP previously filed a citizen's petition with the FDA to remove benzethonium chloride (BZT), a known neurotoxic and cytotoxic substance, from presentations of ketamine intended for intravenous use. The FDA has previously disallowed the use of BZT in hand cleansers and topical antiseptics. A related preservative, benzalkonium chloride has demonstrated corneal and conjunctival toxicity in artificial tears and glaucoma medications, leading to use of preservative-free alternatives. NRXP has filed expert testimony from accredited toxicologists regarding the toxicity of BZT, which is not generally recognized as safe (GRAS) by the FDA. Removal of potentially harmful preservatives from foods is a stated priority in the MAHA report and HHS leadership has additionally targeted preservatives in vaccines. BZT was originally added to ketamine when it was first formulated in the 1970s to maintain stability and sterility using the container closure systems then available. NRx has demonstrated long term stability and sterility with a patented preservative-free formulation using modern manufacturing methods.
Dura Medical Acquisition Completed in Network of Interventional Psychiatry Clinics
On September 8th NRXP announced the closing of its acquisition of Dura Medical. Dura, together with the pending Neurospa TMS and Cohen and Associates acquisitions, are planned to provide a comprehensive service offering to patients at more than 8 locations along the West Coast of Florida. Dura is revenue generating and EBITDA positive.
More on Wisconsin Eagle
Dura delivers a full range of precision psychiatry services for severe depression and PTSD, including Ketamine Therapy and Transcranial Magnetic Stimulation to Veterans and civilian patients.
Second Quarter 2025 Corporate Update
On August 18th NRXP announced financial results for the quarter ended June 30, 2025, and provided a corporate update. As of June 30, 2025, NRXP had approximately $2.9 million in cash and cash equivalents.
The latest NRXP key developments included the following points:
NRXP Drug Development
Grant of expanded Fast Track Designation for NRXP NRX-100 from the FDA for all indications and types of depression and related disorders based on its potential to satisfy an unmet medical need.
Approximately 10-fold expansion of the addressable market to 13 million Americans, compared to the original Fast Track Designation issued in 2017 for bipolar depression alone.
NRXP Filing of Commissioner's National Priority Voucher application for intravenous ketamine (NRX-100).
Submission of draft labeling for NRXP NRX-100 in the treatment of suicidal depression based on the Fast Track Designation received.
Submission of stability data for NRXP NRX-100 to the manufacturing data on file with FDA sufficient to support three years of room temperature shelf stability for NRX-100.
Filing of a patent application for NRXP NRX-100.
Receipt of a PDUFA filing fee waiver from the FDA for NRXP NRX-100.
NRXP filing of module 3 manufacturing data to support a New Drug Application for NRX-101 in the treatment of patients with suicidal bipolar depression and akathisia despite treatment with already-approved medication.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
H.C. Wainright Analyst Report Cites Paradigm Shift in the Treatment of Depression with Suicidality; Assuming Coverage with Buy and $40 Price Target.
Re-Filing of Abbreviated New Drug Application (ANDA) for KETAFREE™, Preservative-Free IV Ketamine.
Notification of US Food and Drug Administration Approval of Suitability Petition for NRx's Proposed Strength of Preservative-Free Ketamine.
Current Ketamine Market Estimated at $750 Million and Projected to Reach $3.35 Billion Globally in 2034.
Dura Medical Acquisition Completed in Network of Interventional Psychiatry Clinics.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
H.C. Wainwright has issued a new Analyst Report on NRXP: "A Paradigm Shift in the Treatment of Depression With Suicidality" Assuming Coverage With a Buy and $40 Price Target. The full report may be accessed at this direct link: https://hcwco.bluematrix.com/links2/secure/pdf/acdd3260-630e-48e6-9c2f-03fbc0be37d6
More on Wisconsin Eagle
- New Article by Roy J. Meidinger – Examines Hidden Hidden Healthcare Kickbacks
- Why Generic Platforms Fail in Emerging Markets: Bettorify Exposes the Gap Between Promise and Reality
- Blogging Pioneer Sherry Bennett Celebrates 29 Years Online - Sharing the Secrets Behind Her 7-Figure Blog Empire
- Koplon Dentistry Elevates Implant Expertise with Advanced CE Course
- i2 Group Acquisitions and Investments in Innovations Deliver 40% Increase in Year-on-Year Bookings
NRXP Re-Files Abbreviated New Drug Application (ANDA) for KETAFREE™, Preservative-Free IV Ketamine
On September 29th NRXP announced the re-filing of its Abbreviated New Drug Application (ANDA) to the U.S. Food and Drug Administration (FDA) for KETAFREE™, its preservative-free IV ketamine formulation, for use in all existing approved indications. The filing follows FDA grant of approval of its Suitability Petition for the NRXP proposed strength of preservative-free ketamine.
The current annual ketamine market is estimated at $750 million, with global demand for ketamine projected to grow to $3.35 billion by 2034. This does not include the widespread use of compounded ketamine by clinics unable to obtain manufactured drug. NRx aims to capture a significant share of the current market. According to a 2021 survey, an estimated 5.1 million Americans had received ketamine for medical uses in their lifetime3, a number that continues to grow with increased clinical focus on this important medication. Ketamine currently faces a severe drug shortage according to the American Society of Hospital Pharmacists. Accordingly, NRXP is seeking priority review from FDA.
NRXP previously filed a citizen's petition with the FDA to remove benzethonium chloride (BZT), a known neurotoxic and cytotoxic substance, from presentations of ketamine intended for intravenous use. The FDA has previously disallowed the use of BZT in hand cleansers and topical antiseptics. A related preservative, benzalkonium chloride has demonstrated corneal and conjunctival toxicity in artificial tears and glaucoma medications, leading to use of preservative-free alternatives. NRXP has filed expert testimony from accredited toxicologists regarding the toxicity of BZT, which is not generally recognized as safe (GRAS) by the FDA. Removal of potentially harmful preservatives from foods is a stated priority in the MAHA report and HHS leadership has additionally targeted preservatives in vaccines. BZT was originally added to ketamine when it was first formulated in the 1970s to maintain stability and sterility using the container closure systems then available. NRx has demonstrated long term stability and sterility with a patented preservative-free formulation using modern manufacturing methods.
Dura Medical Acquisition Completed in Network of Interventional Psychiatry Clinics
On September 8th NRXP announced the closing of its acquisition of Dura Medical. Dura, together with the pending Neurospa TMS and Cohen and Associates acquisitions, are planned to provide a comprehensive service offering to patients at more than 8 locations along the West Coast of Florida. Dura is revenue generating and EBITDA positive.
More on Wisconsin Eagle
- Opening the Virtual City Hall: Revize Unveils a Redesigned Website for the City of Waukesha
- New Book Release: The Tree That Could Not Change
- BayWa r.e. Solar Trade and WHES Announce Distribution Partnership for the European Market: Delivering Smarter Energy Storage
- Fleet Mining Cloud Mining Platform — Latest Guide: Making Bitcoin Mining Safer and More Convenient
- Keebos Launches Crossbody Cases for Every iPhone 17 Model
Dura delivers a full range of precision psychiatry services for severe depression and PTSD, including Ketamine Therapy and Transcranial Magnetic Stimulation to Veterans and civilian patients.
Second Quarter 2025 Corporate Update
On August 18th NRXP announced financial results for the quarter ended June 30, 2025, and provided a corporate update. As of June 30, 2025, NRXP had approximately $2.9 million in cash and cash equivalents.
The latest NRXP key developments included the following points:
NRXP Drug Development
Grant of expanded Fast Track Designation for NRXP NRX-100 from the FDA for all indications and types of depression and related disorders based on its potential to satisfy an unmet medical need.
Approximately 10-fold expansion of the addressable market to 13 million Americans, compared to the original Fast Track Designation issued in 2017 for bipolar depression alone.
NRXP Filing of Commissioner's National Priority Voucher application for intravenous ketamine (NRX-100).
Submission of draft labeling for NRXP NRX-100 in the treatment of suicidal depression based on the Fast Track Designation received.
Submission of stability data for NRXP NRX-100 to the manufacturing data on file with FDA sufficient to support three years of room temperature shelf stability for NRX-100.
Filing of a patent application for NRXP NRX-100.
Receipt of a PDUFA filing fee waiver from the FDA for NRXP NRX-100.
NRXP filing of module 3 manufacturing data to support a New Drug Application for NRX-101 in the treatment of patients with suicidal bipolar depression and akathisia despite treatment with already-approved medication.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: Corporate Ads
0 Comments
Latest on Wisconsin Eagle
- KeysCaribbean Offers 20 Percent Off Seven-Night Stays For Private Home Collection Properties
- Advancing Circular Economy in Automotive ESD Packaging
- Institute for Pet Health Sciences Names Boops Pets 2025 Product of the Year
- Book 2 of "Growing Up Alex" Series for Young Readers Addresses the Challenges of Friendship
- Matthew Cossolotto, Author of The Joy of Public Speaking, Appears on "Get Authentic with Marques Ogden" and "Achieving Success with Olivia Atkin"
- CCHR Exposes Conflicted Psychiatrists Behind Teen Antidepressant Surge
- WIBO Announces Fall 2025 Entrepreneurship Programs to Empower NYC Founders and Small Business Owners
- Local College Student Launches "Cleopatra" App to Make Cleaning Easy for Mercer County Residents
- Wohler announces release of additional Balance Control output tracking for its eSeries in-rack monitor range
- A Milestone of Giving: Ten Percent Group Donates £25,000 to Cure Parkinson's
- Tami Goveia Enters FabOver40, Inspiring Hollywood Legacy for Breast Cancer Cause
- Swidget Launches Luminance™ to Help Schools Achieve Alyssa's Law Compliance
- Growing Demand for EVA Mats Signals Shift in Car Interior Market
- MDRN MUSE Expands Insurance Network Coverage to Include Delta Dental & Cigna
- Hollywood In Pixels Celebrates the 8th Annual Silver Pixel Awards and Announces 2025 Campaign Pixel Winners Los Angeles, CA — Oct
- Physician Calls for States Nationwide to Ensure ADA Compliance in Independent Commissions
- MEDIA ADVISORY - Strengthening Children's Mental Health Across New Jersey
- NumberSquad Launches Year‑Round Tax Planning Package for Small Businesses and the Self‑Employed
- GlexScale launches a unified model for sustainable SaaS expansion across EMEA
- SwagHer Society Launches to Help Black Women Be Seen and Supported